Abstract
Introduction: Mantle cell lymphoma (MCL) is a rare, clinically heterogenous, but mostly aggressive type of non-Hodgkin lymphoma. First line (1L) treatment strategies varied in the past but a chemo-immunotherapy induction regimen containing high dose cytarabine (HiDAC) followed by autologous stem cell transplantation (ASCT) has become the standard of care in young, fit patients with MCL. The benefit of upfront ASCT in the rituximab era has however been questioned following a recent post-hoc analysis of the first randomised study evaluating upfront autologous transplantation in MCL as well as a meta-analysis of seven studies. We therefore evaluated outcomes in our own patient cohort to evaluate the effect of 1L autologous transplantation compared to historical cases that did not receive consolidation.
Methods Patients were identified through our histopathological database and the PROMISE database for patients transplanted at our centre for MCL, between 2000 and 2022. Patients who were on active surveillance or those that received 1L allografts were excluded. We divided patients into 2 groups by age (>66 and <66 years) as per the age cut-off for inclusion in the NORDIC trials. There were however 6 patients aged over 66 years that were included within the transplant group. Survival analysis was performed using the Kaplan-Meier estimator. Progression free survival (PFS) was defined from treatment start date until disease progression or death. Overall survival (OS) was defined as treatment start date to date of death. Data was censored at last follow-up.
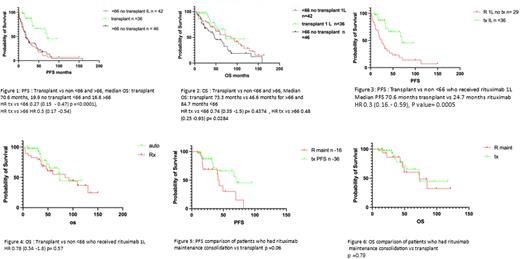
Results : 124 patients were identified with sufficient data for analysis. There were 36 patients who received ASCT consolidation in first remission. 1L therapies included the NORDIC protocol (RMaxiCHOP or RMaxiCHOP alternating with HiDAC), FC+/-R, FCM-R, PMitCEBO, CHOP+/-R, Ibrutinib, R-Bendamustine, clinical trials such as SHINE and ENRICH, as well as splenectomy or radiotherapy. There were 42 patients aged <66 years and 46 patients >66 years who received 1L regimens without transplant consolidation. Of the 42 patients <66 years old, 29 had rituximab as part of induction. Overall, 16 patients received maintenance rituximab without 1L transplant.
In all 124 patients, with a median follow up of 41.25 months, a significant benefit in PFS (median PFS of 70.6 months vs 18.3 months HR 0.25 (0.16 - 0.39), p-value =<0.0001) was observed in favour of patients receiving 1L autologous transplant (Fig. 1) including in those who were <66 (median PFS of 70.6 months vs 19.9 months HR 0.3 (0.15.- 0.47), p-value=<0.0001.)
A significant PFS benefit was also seen when the analysis was restricted to patients treated in the rituximab era (Fig. 3) who were <66 (median PFS of 70.6 months vs 24.7 months HR 0.3 (0.16.- 0.59), p-value=0.0005). When restricting the comparison to patients receiving rituximab maintenance instead of transplant (Fig. 5), although this was a smaller group, median PFS was 70.6 months for transplant vs 43.2 months for rituximab maintenance (HR 0.42 (0.17-1.04), p=value 0.06). Despite the numerical advantage seen in median PFS with 1L transplant, this did not quite reach statistical significance. There was no OS benefit seen with ASCT (Figs. 2, 4 and Fig. 6). In our ASCT cohort there were no deaths <100 days post-transplant.5 of the 6 patients >66 years who had 1L autografts tolerated it well.
Conclusion Our study shows a trend towards reduced benefit with 1L transplant in the rituximab era in eligible MCL patients. While this is similar to the data published by Zoellner et al and Lui et al, there is a notable although not statistically significant, improvement in PFS with 1L transplant consolidation compared to rituximab maintenance in our cohort. In addition, the more recent introduction of rituximab maintenance post 1L autologous transplant and overall survival benefit reported from this approach is not accounted for in our analysis as it requires longer follow up. We conclude on this basis that it is justifiable to continue offering 1L transplant consolidation followed by rituximab maintenance to eligible MCL patients. Prospective clinical trials will hopefully help identify either patients in whom 1L transplant could be omitted, or therapies that may replace 1L transplant.
Disclosures
Nicholson:Amgen: Other: Travel grant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Meeting grant. Cunningham:Celgene: Research Funding; Lilly: Research Funding; 4SC: Research Funding; Bayer: Research Funding; Clovis: Research Funding; AstraZeneca: Research Funding; MedImmune: Research Funding; Roche: Research Funding. El-Sharkawi:AbbVie: Consultancy, Honoraria; Astex: Consultancy; AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Gilead: Honoraria; Janssen: Consultancy, Honoraria; Kyowa Kirin: Consultancy; Lilly: Consultancy; Novartis: Honoraria; Roche: Consultancy, Honoraria; Takeda: Honoraria. Iyengar:Beigene: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: conference support, Speakers Bureau; AbbVie: Other: conference support; Janssen: Speakers Bureau; Kite Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal